
Sodium hydroxide is extensively used in many different industries enabling production of paper, soap, and aluminium etc.
Some applications of chlorine include PVC thermoplastics production, disinfectants, and solvents. Other technologies are under development due to the high energy consumption of the electrolysis, whereby small improvements in the efficiency can have large economic paybacks. Each of those uses a different method to separate the chlorine from the sodium hydroxide. This electrolysis is conducted in either a mercury cell, a diaphragm cell, or a membrane cell. The others are progressively more insoluble in water (K sp is 10 -10, 10 -13, and 10 -16 for AgCl, AgBr, and AgI), reflecting increasing covalency as Δχ decreases.It is the starting point for the chloralkali process, the industrial process to produce chlorine and sodium hydroxide, according to the chemical equation 2 NaCl + 2 H 2 O → e l e c t r o l y s i s Cl 2 + H 2 + 2 NaOH Of these compounds, only AgF is soluble in water and should be thought of as an ionic compound. Our lattice energy calculation overestimates the ionic contribution in the case of the heavier silver halides, but underestimates the covalent contribution. The covalent bonding contribution to the lattice energies of AgCl, AgBr, and AgI makes these salts sparingly soluble in water.Īgain, we can interpret the fortuitous agreement between the calculated and experimentally obtained energies in terms of compensating errors. This reaction is used as a qualitative test for the presence of halide ions in solutions. Should we interpret the good agreement with values calculated from the ionic model to mean that these compounds are ionic? Clearly, this description is inappropriate for AgI, where the electronegativity difference Δχ is only 0.6 (compare this value to 0.4 for a C-H bond, which we typically view as non-polar).Ī drop of siver nitrate solution, when added to a dilute hydrochloric acid solution, results in the immediate formation of a white silver chloride precipitate. However we are still obtaining answers within about 12% error even for AgI. Looking at the table, we see that the error is small for AgF and becomes progressively larger for the heavier silver halides. It is interesting to repeat this exercise for the silver halides, which have either the NaCl structure (AgF, AgCl, AgBr) or zincblende structure (AgI). The errors in this case are only about 1% of E L. The table below shows results of more detailed lattice energy calculations for ionic fluorides in which the van der Waals term is explicitly included.

We can do better by explicitly including the short-range van der Waals attractive energy between ions. The two errors partially compensate, so the overall error in the calculation is small.

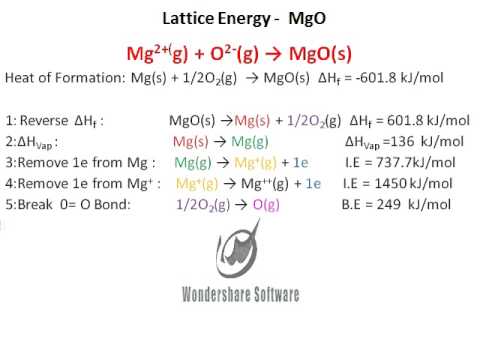
If we underestimate the attractive energy of the crystal lattice, the energy minimization criterion ensures that the repulsion energy is underestimated as well. This is because we used energy minimization to obtain the repulsion energy in the Born-Mayer equation. The result is promising because we neglected the van der Waals term.īut.how did we get away with neglecting the van der Waals term? Here we have to subtract 2RT to convert our cycle of energies to a cycle of enthalpies, because we are compressing two moles of gas in making NaCl(s) and PΔV = ΔnRT, where Δn = -2.Įxperimentally ΔH f for NaCl is -411 kJ/molīecause all the other numbers in the cycle are known accurately, the error in our calculation is only about 15 kJ (about 2% of E L).


 0 kommentar(er)
0 kommentar(er)
